© 1979 Robert A. Freitas Jr.
All Rights Reserved
 |
| The bioenergetic challenge |
Why are xenologists so concerned about bioenergetics? Bioenergetics means, simply, the study of biological energy. The engine of life, as any machine, needs a supply and a flow of energy — chemical, electrical, thermal, or whatever — to keep it running. If power is suddenly cut off, both mechanical and biological machines soon grind to a halt.
|
Fundamental requirement of life: a flow of energy from a source to a sink |
The thermodynamic definition of life discussed in an earlier chapter — that living systems "feed on negentropy" and thereby manage to maintain themselves against the universal drive to disorder mandated by the Second Law of Thermodynamics — demands a flow of energy from a source to a sink. This requirement is so fundamental to the basic character of life itself that we may confidently predict that bioenergetics will be a favorite discipline among alien zoologists and physiologists. But can we be as certain about the specifics?
The Viking Lander biology package assumed so. The Pyrolytic Release device tested for photosynthetic activity on Mars, and the other two experiments sought evidence of respiration and simple metabolism. But were these assumptions reasonable? Must lifeforms evolving under alien suns on distant worlds conform to Earthly patterns and cycles?
| ET photosynthetic animals |
ETs will have met the bioenergetic challenge in many diverse and unexpectedly clever ways. Each new race independently must have evolved intriguing and totally unique methods for absorbing, storing, distributing and regulating energy. While we don’t know for certain if extraterrestrial photosynthetic animals are possible elsewhere in our Galaxy, or if alien bloodstreams will run red, green or blue, or whether in some distant corner of the universe there exist "biological refrigerators" which can stabilize body temperatures on sweltering worlds as hot as blast furnaces, xenologists cannot resist the temptation to pose these and other fascinating questions.
|
All life on Earth ultimately depends upon one of two sources:
Photons from Sol (photosynthetic organisms) and Chemical energy (all nonphotosynthetic terran lifeforms). |
Active life requires a flow of energy between a source (a region of high energy) and a sink (a region of lower energy). To use heat energy, for example, a difference in temperature between two points in space must be maintained. A steam engine works not because it is hot, but rather because the boiler is hotter than the condenser.
| Plant life |
How does plant life fit into this scheme? It has been said that the only reason photosynthesis works at all is that the surface of the sun is at 6000 K, whereas the surface of the Earth (and its plant life) is only at 300 K. Photons emitted at the higher solar temperature travel through space to this planet, enter the chlorophyll molecule and power the plant’s metabolism. Later, photons of waste heat, a form of degraded energy, are radiated off at the far lower planetary surface temperature.
| Animal sources and sinks |
Animals too need sources and sinks. The food they eat is burned by the oxygen they breathe, and this constitutes a useful source of energy. The external environment, by accepting bodily waste heat, serves as a sink.
All life on Earth ultimately depends upon one of two sources: Photons from Sol (photosynthetic organisms) and chemical energy (all nonphotosynthetic terran lifeforms). However, various other possibilities have been suggested for hypothetical alien beings on other worlds.
One widely discussed alternative involves the evolution of life on so-called "starless planets."128,816 Such worlds, if they exist, lie in the dark plumbless abyss of interstellar space far from the coddling embrace of any friendly star. Were the object large enough, say, a massive jovian or super-jovian, it might be warmly self-heating with a tepid surface crust.
Of course, we know that heat alone will not power a living organism. And it is difficult to imagine how to establish a flow of energy in an environment heated to some relatively fixed, unvarying temperature. Most writers have ruled out life on starless planets on this basis.18,22,714
Dr. Thomas Gold at Cornell University disagrees. If we consider the surface of a starless planet as a source at 300 K, he points out, then all we need to do is find a sink somewhere at a lower temperature to establish a life-giving flow.
Space is very cold, only about 2.7 K. If this or something very close to it could be used for the energy sink, then biological thermodynamic efficiencies approaching those of terrestrial photosynthesis might in principle be possible.22
|
Many other imaginative and exotic energy systems have been
postulated by various writers, including geothermal heat and volcanism, piezoelectricity, solar wind ions, planetary magnetic fields, atmospheric electricity (lightning), and radioactive decay (fission). |
Extending this idea just a bit further, Gold suggests that some alien lifeforms may base their processes on a thermal gradient in time rather than in space. Imagine a uniformly heated environment in which there was a slow but regular diurnal temperature variation. Usable bioenergy could be extracted through the use of a chemical system which coupled only to the equilibrium state established at each extreme.
At the hottest extreme, certain reactions might take place which stored energy in chemical form. This energy would then be released only when the temperature swung down to the coolest extreme. In this scheme, the source and sink are no longer coextensive in time. As temperature fluctuates, the surroundings would be first the source, later the sink, and so on.
Many other imaginative and exotic energy systems have been postulated by various writers, including geothermal heat and volcanism, piezoelectricity, solar wind ions, planetary magnetic fields, atmospheric electricity (e.g. lightning), and radioactive decay (fission power).
J.W. Ycas has come up with a novel form of energy transduction, to which he has given the formidable appellation "palirrhotrophy."2379 His organisms, should they exist, are powered by chemical osmosis. A flow of bioenergy — an "osmotic current" — is established "by exploiting the rhythmic variations in salinity which occur in the estuarine environment." As the palirrhotrophic lifeform is periodically flushed, first with salty seawater and later with upriver freshwater, energy is pumped into its system osmotically.
Such creatures might exist on a predominantly watery world, one with a large moon or moons and a fast rotation to make the tides frequent but brief. A tropical climate would ensure plenty of rainfall and a bountiful source of freshwater, and high gravity would cause mountain water runoff to cut deep channels and fjords to the sea — a viable niche for palirrhotrophic ETs.
 |
| Mechanical energy |
Another distant possibility is the use of mechanical energy. The waves, winds or tides might be harnessed to power a shore-dwelling alien creature. A slowly rotating planet with a massive moon in a fast orbit would have plenty of mechanical wave energy available at the surface. Yet organisms would find themselves without sunshine for such long night-time stretches that they might find it useful to evolve a biomechanical energy system as an auxiliary power supply.
| Thermoelectric organism |
A similar proposal is that extraterrestrial lifeforms might be able to use the internal heat flowing up though the surface of a terrestrial world. Unfortunately, even on a world as far removed from the stellar campfire as cold, distant Pluto, the sun out radiates internal planetary sources by nearly two orders of magnitude. A more viable proposition, perhaps, is the concept of the thermoelectric organism.607 On a planet with thin air, located close to its star, the temperature differential between direct sunlight and shade might be sufficient to adequately power an alien biochemistry.
| Thermonuclear lifeforms |
A few hardy souls have even suggested thermonuclear lifeforms. At a meeting of the British Interplanetary Society back in 1948, Olaf Stapledon proposed that the fusion power of the sun might conceivably be harnessed as an alternative to biochemical processes.556 Although Isaac Asimov has used this idea in the context of a small, planetbound animal,94 such a power supply might be more apropos for electromorphs akin to Hoyle’s Black Cloud.*
* It is interesting to note that Sol, the only nearby entity we know of that uses fusion power, has an overall energy output of only about 0.0002 watts/kg. The human body, on the other hand, operates at a whopping 2.0 watts/kg, about four orders of magnitude higher than the sun!
|
|
|
Despite that many energy schemes noted above, not all processes theoretically permissible under the Second Law of Thermodynamics are commonly or easily available to living organisms. The methods for the performance of useful work used, say, in modern industry are generally not utilized by Earthly lifeforms. For instance, changes in temperature such as might result from combustion or nuclear reactions are not found in biology. Instead, the creatures on this world uniformly may be characterized as "chemodynamic machines," operating by chemical rather than by thermal energy.
This is not a serious restriction. As Dr. E. Broda at the Institute of Physical Chemistry in Vienna points out, nutritionists have observed that "surprisingly high yields of useful energy can be obtained from food." Comparing chemical and thermal systems, Broda continues: "With a yield of 25% as observed, the {equivalent} temperature difference works out as 105 °C. Hence, if the body was a heat engine, local temperatures of at least 310 K (body temperature) + 105 K (food-conversion temperature) = 415 K would be needed."1013
While we recall that arguments on the basis of temperature alone cannot rule out the possibility of life, this example serves to illustrate the superior competitiveness of chemodynamic as opposed to strictly thermodynamic energy systems. We conclude, perhaps somewhat chauvinistically, that nonexotic chemical energy systems will normally be the method of choice for the majority of extraterrestrial lifeforms. Naturally, the most convenient and abundant source of usable energy for most ETs will be their sun.
|
It is difficult to imagine an easier or more elegant
solution to the fundamental bioenergetic problem. |
The process of energy utilization by a living creature is its metabolism.
Given the problem of designing a metabolic system, starting from the sole assumptions that:
- a chemical framework
- powered by sunlight
must be used, we quickly arrive at two logical conclusions.
| Autotrophs |
|
Autotrophs Harvest energy:
Heterotrophs Pirate energy-riches:
|
First, the simplest organisms in a planetary ecology will be those capable of tapping the given energy source directly. These lifeforms accumulate energy from photons received from the sun, absorb any needed inorganic matter that happens to be lying around, and put the two to work in an integrated biochemical system. Because they are able to harvest energy straight from the original source all by themselves, such creatures are called "autotrophs."
| Heterotrophs |
Second, we might imagine another kind of lifeform which cannot tap the energy source directly. This class of organisms is either too lazy or too incompetent to manufacture its own food. So what powers them? Instead of patiently accumulating solar energy, these larcenous "heterotrophs" pirate energy-riches from the complacent autotrophs. Since there is no honor among thieves, we would also expect to find heterotrophs stealing energy from each other as well. An entire chain of robbery would develop, with the strong taking from the weak, the stronger taking from the strong, and so forth.
With a few minor variations, this is the basic scheme of life on Earth. The autotrophs are our plant life, which take up carbon dioxide and convert it to carbohydrates and other energy-rich goodies. The heterotrophs are the animals.
Clearly, the organization of an ecology into two major groupings (producers and consumers) is not at all arbitrary but follows logically from the twin assumptions stated earlier. It is difficult to imagine an easier or more elegant solution to the fundamental bioenergetic problem. Although other ecological systems may exist, the dual autotroph/heterotroph arrangement is probably the preferred technique for chemically-based, solar-energized metabolizers.1428
Each year about 150 billion tons of carbon are taken in by the autotrophic plants on this planet and are combined photosynthetically with some 25 billion tons of hydrogen (split from the oxygen in water) to make carbohydrates. In the process, 400 billion tons of oxygen are set free. On the average, a typical molecule of carbon dioxide wends its way through the system once every 200 years; each O2 cycles less frequently, perhaps once every 2000 years.997
Of course, it is not absolutely necessary for alien autotrophs and heterotrophs to participate in a carbon cycle biochemistry powered by the breakdown of water to oxygen. While the photosynthetic process itself is so simple as to suggest a certain measure of universality, there is nothing sacred about which chemicals are recycled. In fact, there are a number of other systems in use today right here on Earth.
| Sulfur cycle as alternative |
One alternative to the photosynthetic H2/O2 cycle of which humanity is a part is the H2/H2SO4 process of the sulfur bacteria — an entirely different oxidation-reduction system than the one we use. Purple sulfur bacteria take in hydrogen sulfide (H2S) and oxidize it to sulfuric acid (H2SO4). Desulfovibrio, another class of sulfur bacteria, completes the cycle by reducing the acid back to the original hydrogen sulfide gas.
Many other systems are in use on Earth besides this "sulfur cycle." There is an H2/H2O cycle, a CH4/CO2 cycle, an NH3/N2 cycle, and so forth. Microorganisms on this planet are capable of metabolizing such peculiar and diverse substances as selenium, iron sulfide, arsenic, thiosulfate ion, cyanides, and methanol. But the main hangup with using any of these exotic non-oxygenic systems to power large extraterrestrial organisms is their relative inefficiency.
Most are at least an order of magnitude less energetic than the water/oxygen cycle which dominates the biochemistry of Earth.
Because they are so woefully inefficient, non-oxygen-cycle lifeforms are significantly out-competed in most terrestrial environments and "have been driven to the fringes of life-as-we-know-it."1390,1651 Nevertheless, there have been many valiant attempts to design viable extraterrestrial ecologies around various alternatives, notably by Asimov,1358 Clement,292 Glasstone,72 Mitz,1424 Salisbury,1658 and Vishniac et al.313
|
Porphyrins are very simple ring-shaped molecules
which have been produced in many prebiotic synthesis experiments,and which are believed capable of autocatalyzing their own production. Once formed, the porphyrin ring has enormous stability against decomposition. This may help to explain why these substances are so widely distributed on Earth today. |
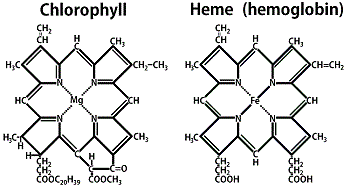
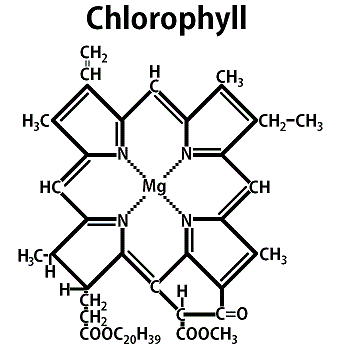
If photosynthetic activity is extremely useful if not essential on other worlds, what is the best way to do it? Although there are many other molecules at work, chlorophyll predominates on Earth. Chlorophyll, the green active pigment in plants, is a member of a general class of carbon compounds known to biochemists as porphyrins.
Porphyrins are very simple ring-shaped molecules which have been produced in many prebiotic synthesis experiments,1590 and which are believed capable of autocatalyzing their own production. Once formed, the porphyrin ring has enormous stability against decomposition. This may help to explain why these substances are so widely distributed on Earth today.
The porphyrin pigment chlorophyll has a single magnesium atom located in dead center. The exact function of this metal atom has yet to be clarified, but it is believed to play a crucial role in trapping and utilizing the energy of incoming photons used in photosynthesis.
If alien autotrophs use porphyrins too, will they be restricted to green, magnesium-based chlorophyll?
| The case for magnesium porphyrins |
A few have argued that we should consider only the most abundant metallic elements in Earth’s crust — say, the top 99% in abundance — as candidates for the central atom.2374 If we buy this assumption, then a fairly good case can be made for the exclusivity of magnesium porphyrins in any water-solvent oxygenic biochemistry.1423,2399
Of course, this is only a plausibility argument — one which utterly fails if alternative liquid media (other than water) are considered. And even in water, despite the many points in favor of Mg, some doubt remains. Other possibilities may be open to ETs.
While photochemists have so far been unable to produce a substitute porphyrin complex "which involves relatively large storage acts" per photon of energy absorbed,993 it is well-known that zinc (Zn) porphyrin complexes are capable of undergoing reversible photochemical oxidation-reduction reactions similar to those exhibited by Mg-porphyrins.1422,1423
One chlorophyll near-analogue, called zinc tetraphenylporphyrin, has shown weak photoactivity.993 Other zinc porphyrins, although admittedly rare on this planet, have been found in several organisms including Rhodopsuedomonas apheroides, the diphtheria bacillus, various mammalian organs, and in leaf tissue homogenates.994,1069 Copper porphyrins have also been found in the diphtheria bacillus, and other substitutions using nickel, cobalt, or manganese are remotely possible but seriously questioned.1422,1442
But perhaps we are being overly restrictive. What, after all, is so magical about the porphyrins? True, they arise in prebiotic experiments, and true, they are relatively simple molecules and they get the job done. But maybe there exist other equally suitable substances that could stand in for chlorophyll in alien plant biochemistries.
It seems difficult for many to believe that porphyrins are the best-suited class of molecules for the photosynthetic function. In fact, according to Bernard Pullman of the Institut de Biologie Physico-Chimique, University of Paris in France, "it certainly is not."315 George Wald, Carl Sagan, and countless others have pointed out that chlorophyll actually absorbs light rather poorly in the green portion of the spectrum — paradoxically, the very region where solar radiation is most intense. It is likely that a variety of dyes other than chlorophyll could have been used by plants.
| Terran photopigments |
Several alternative pigments are known in terrestrial biology to participate in the process of light absorption. For example, the carotenoids — found in many species of bacteria, algae, and higher autotrophs — absorb primarily blue light (which has more energy per photon) and thus are red, orange, or yellow in color. Carotenoids have no metal atoms and contain no porphyrin-like substances. Another category of terran photopigments are the phycobilins, which give both the red and the blue-green algae their distinctive, vivid color.
In the purple "halobacteria" (salt-loving), chlorophyll is entirely replaced by another Earthly photosynthetic pigment called bacteriorhodopsin. This substance has a deep purple color and is chemically related to rhodopsin, the photosensitive pigment called "visual purple" found in the rods of all mammalian eyeballs.
Research has suggested that this pigment may be selectively more advantageous in certain specific environments. This is particularly true under conditions of intense sunlight, elevated temperature, high salinity, and low oxygen concentrations.2402 As a possible photosynthetic agent for extraterrestrial plant life, purple bacteriorhodopsin is less efficient (by one-third) but chemically simpler than chlorophyll.
| Oxides possessing high photosensitizing activity |
Going still farther afield, why must complicated organic compounds be used at all? It has been amply demonstrated that the oxides of titanium (white), tungsten (canary yellow), and zinc (white) all possess high photosensitizing activity in oxidation-reduction reactions comparable to the activity displayed by chlorophyll. And these particular pigments are known to store light-energy in stable terminal products.2374 This may be useful for biology.
| Organic photocells |
Finally, it is also well-known that many carbon-based organisms are capable of utilizing silicon and germanium to varying degrees. Why could not alien autotrophs, instead of sporting leaves impregnated with chlorophyll, sprout thin platelets of "organic photocells" analogous to the solar cells used by NASA to power spacecraft? Water could be split up by some electrolytic process, and the hydrogen thus liberated incorporated into useful energy-rich molecules.
Any of these substances could serve as photosynthetic pigments for alien plants. When human explorers reach out to other worlds, they may discover beautiful white, blue, red, yellow, orange, purple, glittering steel-gray — yes, even green! — landscapes of thriving vegetation.
|
Given optimum shape and plausible
environmental conditions, autotrophic turtles are quite possible. |
We have seen that photosynthesis is a highly useful means for collecting and storing solar energy. But only plants have been discussed. Could "animals" use this technique as well? This idea has cropped up from time to time in science fiction, so it is worthwhile to deal with it briefly here.
The basic idea is that it might be possible to design an alien metabolism falling somewhere between pure autotrophism and pure heterotrophism. The microscopic flagellate Euglena could be a possible ancestor of such creatures. This tiny microbe feeds both by chlorophyllic photosynthesis (like a plant) and by direct absorption of organic food (like an animal).
| Plant men |
But when it comes to larger organisms, many writers have been unable to conceive of plausible autotrophic animals. Usually it is alleged that "plant men" are impossible because they would be incapable of collecting enough energy fast enough, and that the only remedy for this failing is to become a sessile, vegetable-like being, perhaps akin to a tall, green-skinned saguaro cactus with corrugated skin and large, leafy limbs. But is this really true?
|
Water breathers, to inhale the same amount of
oxygen per minute as a lunged creature, must expose their internal environment to a heat sink with an effective capacity roughly 100,000 times greater than the equivalent amount of air. It is for this reason, xenologists believe, that warm- blooded aliens will almost certainly not have gills. |
The total power requirement of the typical mammal is roughly 3.4M3/4 watts, where M is the mass of the animal in kilograms.1662 The energy received from the sun is a fixed amount, and normal plant efficiencies range from 1-10% in normal light. From these values it is easy to calculate that the maximum size of a chlorophyllic autotrophic mammal on Earth is a small fraction of one millimeter. Moving the planet closer to the sun or raising the photosynthetic efficiency doesn’t help much, either.
But all is not lost! Reptiles, for various reasons, often consume as much as an order of magnitude less energy than mammals of comparable size. Taking this into account, we discover that reptilian autotrophs may be as large as 30 centimeters wide. Given optimum shape and plausible environmental conditions, autotrophic turtles are quite possible.
| Solar-powered avians |
Small autotrophic aerial insects or birds may also be possible on worlds where sizable wingspans are aerodynamically feasible. Energy could be absorbed by chloroplasts locked in the thin skin of the wings. Solar-powered avians may patrol the skies of other worlds high above the surface vegetation, thus escaping certain death under a dense and dimly-lit forest canopy. If their planet rotated slowly enough, they could probably glide sufficiently fast to follow the sun. They could keep their life-giving photoproductive wings in perpetual daylight. Since autotrophic avians would rarely have to stop to hunt for food, they could spend virtually their entire lives engaged in such travels.
| Floating marine species |
Another possible autotrophic animal would be a floating marine species, a pancake-thin stingray-like affair skittering across the surface of the sea. These creatures could grow to enormous sizes, not having to waste much time on hunting or predation. Their main concern would probably be fending off hungry heterotrophic thieves.
|
Fermentation: an anaerobic (oxygenless) reaction leaving most of the energy behind Respiration: in this reaction, the full amount of energy stored in the carbohydrate fuel is recovered |
But Euglena and green hydras aside, plant-eating is the way of life for all animals on Earth. Two broad classes of heterotrophic energy utilization may be imagined.
- First, the rich foodstuffs plundered from plants could be broken down directly for energy. This simple technique, probably used by the earliest protobionts and retained today by the yeasts, is called fermentation. A typical fermentation reaction in which carbohydrate is decomposed looks something like:
- C6H12O6 —> 2C2H5OH (ethyl alcohol) + 2CO2 + 74,600 joules
- This anaerobic (oxygenless) reaction leaves most of the energy behind, locked up in the two molecules of ethyl alcohol — which are discharged as poisonous wastes.
- The second alternative is to employ a powerful oxidant drawn from the environment (e.g. oxygen, chlorine, fluorine, etc.) to biochemically "burn" the carbohydrate fuel. Most animals on Earth today are powered in this way, using oxygen as oxidant, called respiration. In a respiration reaction, the full amount of energy stored in the carbohydrate fuel is recovered:
- C6H12O6 + 6O2 —> 6H2O + 6CO2 + 2,820,000 joules
About one and a half orders of magnitude more energy are released during respiration than during fermentation, at least with carbohydrate fuel.
As a result, many have concluded that animals powered by fermentation cannot be very advanced. This is of interest to xenologists, because if true, it sets a limit to the complexity of evolutionary processes in reducing environments — such as those on the primitive Earth and gas giants like Jupiter and Saturn. Carl Sagan sounds a note of caution:
This is an unimaginative conclusion. There may be more energetic foodstuffs available elsewhere; or the organisms there may eat at a faster rate than do organisms here; or their metabolic processes may be correspondingly slower. It is premature to infer that every planet populated with higher organisms must have an {oxidizing} atmosphere.20
However, there appears to have been great selective advantage for those lifeforms able to metabolize powerful oxidants. Clearly, in any biochemical system, respiratory organisms will obtain far more energy from a given quantity of food than others who rely solely on fermentation. The invasion of land on our world less than an eon ago was immediately preceded by the evolution and rapid deployment of respiratory mechanisms throughout the animal kingdom.2404,2405 Respiration would seem to be the metabolic process of choice for highly active, mobile organisms.
On Earth, three basic designs for respiratory organs have emerged over the eons: Tracheae, gills, and lungs.
| Tracheae |
The first of these is used by insects, worms, and other small creatures. An insect does not really breathe, as we understand the term. The system is essentially a passive one. Oxygen is not carried to the muscles by circulating blood, but rather by a network of branching air tubes called tracheae. Insects introduce oxygen into the interior of their bodies solely by diffusion (sometimes assisted by weak abdominal pumping spasms) — a far slower and less efficient technique than an active, forced-flow oxidant circulation system.
This tends to limit the size of tracheal breathers. Although this passive system might well serve much larger organisms on a planet with high atmospheric pressure (of oxidant),* on Earth insect bodies must remain fairly small to be efficient.89,1730 The largest alive today are the tropical beetles which grow as long as 15 cm; and while tropical dragonflies and centipedes during the Carboniferous Period (and the Devonian Period sea scorpion Pterygotus) often achieved lengths up to 150-180 cm, they were still stuck with attenuated cylindrical bodies no more than a few centimeters in diameter.722
* With much denser air, insects could be somewhat larger than we know them. Flowers might also be larger, broader, more colorful, in response.89
|
Breathing by water seems to present
more problems with fewer rewards than air breathing. |
If an animal can’t wait for oxidant to drift lazily through tracheae to replenish its cells, it can forcibly pump it there using a powerful circulatory system. This is an active system, in contrast to the passive mode of insect breathing.* Circulatory fluid may be enriched with the vital oxidant in one of two ways: Diffusion from a liquid medium (gill breathing), or diffusion from a gaseous medium (lung breathing).
| Water breathers |
Breathing by water seems to present more problems with fewer rewards than air breathing. Since the liquid has a far higher density and viscosity than air, it takes more energy to ventilate a gill than a lung. This difficulty is further aggravated by the simple fact that water equilibrated with the air above contains only 3% as much oxygen in solution. Hence, the gilled animal must pump a lot to breathe a little.
| Lung breathing |
Oxygen diffuses into the respiratory organ about a million times faster in air than in water. Water breathers also have less control over the flow of vital ions between body and environment, which can be quite hazardous.
| Heat loss |
Perhaps the most serious drawback to the use of gills is the problem of heat loss. Water breathers expose their blood to an external fluid of comparable heat capacity, while air breathers encounter a thin gaseous medium with a heat capacity some 3000 times lower than that of their circulatory fluid. Hence water breathers, to inhale the same amount of oxygen per minute as a lunged creature, must expose their internal environment to a heat sink with an effective capacity roughly 100,000 times greater than the equivalent amount of air. It is for this reason, xenologists believe, that warm-blooded aliens will almost certainly not have gills.724
* As a general rule, blood circulates continuously throughout the body. However, in a few animals (notably the annelid worms) there is no such throughput circulation. Instead, bodily fluids undergo a periodic ebb-and-flow cycle, a kind of "tidal irrigation" of the cells.
It has been suggested that the color red is used to signify danger in so many human societies because it is the color of blood. We associate red with blood and bleeding, which in turn are associated with pain, bodily injury, or death. If the blood of ETs is, say, green (Figure 10.1) instead of red, one writer speculates, they "would think green a quite natural index of danger and be amused at the idea of using red."2552
|
Human heart:
|
Virtually all higher organisms on Earth circulate body fluids of one form or another. The grasshopper has a circulatory system involving blood and many hearts — though it is used only for food and waste, and not oxygen, transport. The earthworm has an advanced pumping network with five pairs of hearts, and the squid has two pairs — one at each gill to circulate fresh oxygenated blood, the other pair for returning spent blood. In the human body, a heart weighing about half a kilogram pumps 8000 liters per day through approximately 96,000 kilometers of vessels and capillaries.
One of the most important functions of circulatory fluid is to deliver oxidant to the organism’s cells, by dissolving more of it than would normally be possible in plain water. On Earth, where gaseous oxygen is the most common oxidant in general usage, the utility of blood in this regard is greatly enhanced by the presence of pigments. These pigments are able to combine "reversibly" with O2, picking it up in lung or gill and ferrying it along to the cells. For instance, pigments found in mammals typically can carry some 250 cm3 O2 per liter of blood, as compared to a mere 5 cm3 O2 that can be dissolved in a liter of ordinary seawater at the same temperature.
| Oxygen transport via pressure differential |
The way oxygen transport works is rather clever. Nature has discovered that certain substances will chemically combine with oxygen at high partial pressures, but that if the pressure is lowered past a certain point, the gas is let go again. By maintaining a high partial pressure in the respiratory organ of an animal, and a comparatively low pressure within its cells, O2 may be picked up by the blood pigment in the lungs and later forced to give it up when it reaches the body tissues.
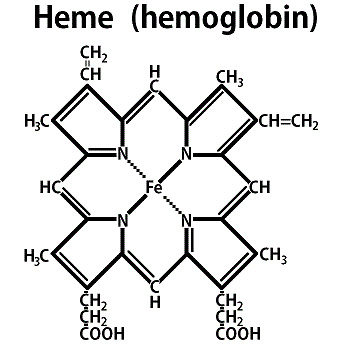
The most successful such pigment on this planet is hemoglobin (Hb), a member of the same class of porphyrins to which chlorophyll belongs (Figure 10.2).
A hemoglobin molecule consists of a porphyrin ring with a central iron atom (heme), hooked to a giant clump of protein called globin.
Hemoglobins are present throughout the entire animal kingdom, in all vertebrates (except a few Antarctic ice-fish1691) and in the circulatory fluids of many invertebrates as well (annelid worms, many arthropods, and some echinoderms, mollusks and crustaceans).
|
Figure 10.3 Respiratory Pigments for Alien Bloodstreams |
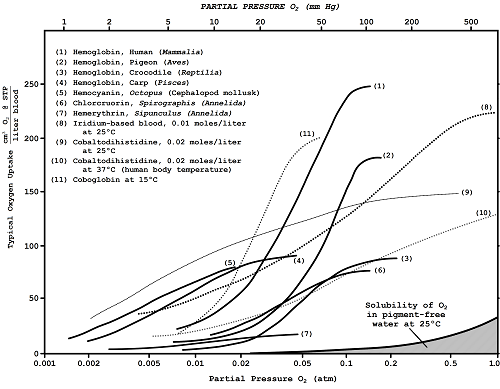 |
Hemoglobin remains the most efficient oxygen-carrier known. For the better part of this century, chemists have labored in vain to create other substances that could do a better job. Nevertheless, several other pigments have been discovered and utilized in nature.* These, while not as efficient as hemoglobin, still are able to serve as adequate oxygen-transporters in certain environments (Figure 10.3).
| Hemocyanin |
The copper-containing pigment hemocyanin, second in breadth of distribution only to hemoglobin, is found in the blood of various mollusks and arthropods.195,723 Unlike human blood, which is bright red in the arteries (oxygenated) and dark red in the veins (deoxygenated), hemocyanin blood is a beautiful blue in the arteries and crystal—clear colorless like water in the veins.
| ET blue bloods |
Hemocyanin is always found floating freely in the blood plasma, rather than being trapped inside corpuscles as are the relatively smaller molecules of Hb. This copper-based, proteinous, non-porphyrin blood pigment is only about 25% as efficient an oxygen carrier as hemogIobin.734 Might true extraterrestrial "blue bloods" exist? On a world with very high surface pressures and abundant oxygen we might suspect that the higher efficiencies achieved by Hb might not be needed for survival. Hemoglobin might then never have evolved at all.
* Most animals with colored blood (except insects) obtain that color primarily from the respiratory pigment. Naturally, since substances of all kinds may be dissolved in the circulatory fluids of an animal, the possibility of blood of any color cannot be positively ruled out. However, in most respiratory terrestrial species it remains true that the blood is vividly colored according to the particular oxygen transport pigment used.
Another free-floating respiratory pigment found in various tubular annelids (Polychaeta), also only about a quarter as efficient as Hb, is chlorocruorin.723 Solutions of this iron-based pigment are green in natural dilute solutions, but in higher concentrations become vivid red in color. The blood of one species, Serpula vermicularis, is remarkable in that it is a dual hemoglobin/chlorocruorin pigment system.
| Hemerythrin |
Hemerythrin is iron-containing proteinous pigment found in the blood of certain bottom-dwelling marine worms (nematodes, annelids) and brachiopods. It serves as a reversible oxygen carrier, but is far less efficient than hemoglobin. Blood containing this pigment is a bright pink or violet when oxygenated, but turns colorless by the time it reaches the veins. A small molecule like Hb, it must be confined to corpuscles in the bloodstream rather than allowed to float about freely.
| Vanadium chromagen |
Another pigment is called vanadium chromagen.1650 This is found in the blood of sea squirts, ascidians, and tunicates. Confined to tiny corpuscles known as vanadocytes, the blood containing these tiny packets is usually apple-green in color but can also be found in blue and orange varieties, presumably due to the presence of different oxides of vanadium.1070Although still questioned by some, vanadium chromagen appears to take up and release O2 freely in acidic solutions.
There are a few others known to biologists, such as the copper-based porphyrin found in the wing feathers of Turacus indicus and the occurrence of a manganese-based porphyrin in the blood of the mollusk Pinna squamosa (called "pinnaglobin," a brownish pigment), whose respiratory functions have not yet clearly been established.
Lifeforms on other worlds could use various combinations or varieties of any of the above to get oxygen to their cells. Their blood could take on virtually any color, as we have seen. But does the inventory of natural techniques on Earth exhaust the possibilities?
Not by a long stretch. Despite the undisputed fact that reversible oxygen binding is a comparatively rare biochemical property, there are many alternatives never seen by nature. For more than half a century now, chemists have been reporting the synthesis of Hb-like molecules, not occurring in nature, capable of reversible combination with oxygen.734 For example, a simple iron-indigo compound seems to work quite well.2411
Porphyrin complexes of dozens of metallic elements have been studied carefully in this regard.914,918,1068 Chemists have discovered suitable manganese and cobalt complexes unknown in terrestrial respiratory systems. Since early in this century, in fact, a wide range of cobalt histidines and "coboglobins" have been investigated in depth. (See especially the work of Hearon et al.,914 Hoffman and Petering,916 Martell and Calvin,1067 and Michaelis.915,2414) They appear most promising for use in possible alien biochemistries. There is some precedent for this on Earth: Vitamin B-12 is a cobalt-based porphyrin.
Coboglobin blood would be colorless with a faint pinkish tinge when loaded with oxygen, but in the veins would take on a dark yellow or deep amber color. Since coboglobin protein is a lightweight molecule like hemoglobin or hemerythrin, we might expect ET blood to carry the pigment in corpuscles.*
| Irreversible oxygenation |
After many cycles of use, a molecule of coboglobin gets old and "tired."
It loses its ability to reversibly bind oxygen. While this aging takes weeks in the case of normal human hemoglobin, less than a day is required for coboglobins to poop out. As this "irreversible oxygenation" sets in, the pigment changes color dramatically from amber to deep pink.
But this should pose no insuperable problem for ETs. We know that millions of human blood cells are broken down and rebuilt each minute of our lives. It is not implausible to suggest that alien organisms may have evolved a more efficient biochemical apparatus for the recycling and reconstitution of blood pigment than have Earthly animals.
It is doubtful that the relative scarcity of cobalt compared to iron will rule out the existence of coboglobins in alien blood. While the element is about 1/100 as abundant as iron in the seas, it is approximately as abundant as phosphorus, chlorine, and potassium, all of which are common in mammalian biochemistry. And the two elements copper and vanadium — used by terrestrial lifeforms in the O2-transporting blood pigments hemocyanin and vanadium chromagen — are almost an order of magnitude scarcer than cobalt in the cosmos and are of comparable abundance on planetary surfaces.**
* Like hemocyanin, hemerythrin and coboglobin are not destroyed by carbon monoxide as is hemoglobin in human blood. Organisms with these kinds of blood pigments thus would not be poisoned by the gas, as humans are.
** Cobalt, not iron, may lay claim to the simplest oxygen binding molecule on record. Cobalt forms a complex with ammonium hydroxide (ammonia dissolved in water) which is capable of reversible behavior. The carrier molecules have a mean lifetime of only a few hours, so must be rapidly recycled much like coboglobin.914
Another interesting though less likely possibility is iridium-based blood. One simple compound with an absolutely horrible name (chloro-carbonyl-bis(tri phenylphosphine)-iridium) has recently been shown to undergo reversible oxygenation.919,920 This substance is insoluble in water and other polar media such as liquid ammonia and alcohols, but this presents no barrier to its use in blood. The vanadium chromagen found in ascidians is also insoluble in water.
In solution, the compound takes up one atom of oxygen per molecule to change from brilliant yellow to sullen orange. The reaction is not quite as fast as with the cobalt complexes, so a more convoluted lung would be necessary.
| Pigment for a dimly lit world |
In the oxygenated condition, the iridium-based blood of extraterrestrials would have to be protected from light because it is very photosensitive. The pigment slowly decomposes over a period of days or weeks when exposed to strong light, gradually changing color from orange to green and finally to a deep bluish-black. Such aliens would therefore either have very dark skin, or would inhabit a dimly lit world. (In the absence of light, the molecule is stable for years.)
The iridium complex has one additional property which is extremely fascinating to xenobiologists. In addition to oxygen, the molecule is also capable of reversibly binding hydrogen as well!
Many other reversibly binding compounds can probably be found if chemists search for them diligently. And we haven’t even really considered the possibility of ETs using oxidants other than oxygen. To date, chemists have scarcely considered reversible chlorine- or sulfur-binding molecules which might serve as respiratory pigments in the bloodstreams of truly alien extraterrestrials.
|
It was quickly discovered
that it was of tremendous advantage to be able to run the biological machinery at top speed in virtually all circumstances. Cold-blooded animals are not really cold
but merely biologically unregulated. |
About 20 joules of energy are released for every cm3 of oxygen consumed in respiration. In reality, however, only a fraction of it can be utilized for activity and useful work. There is much waste heat.
A major problem experienced by extraterrestrial creatures will be controlling the generation and distribution of this excess energy within their bodies.
| Cold-blooded |
Of course, an organism might simply choose to ignore the problem, hoping that the environmental temperature extremes don’t become so great as to make life impossible. These are the "cold-blooded" animals, which are not really cold but merely biologically unregulated.
There are many examples on Earth. Sea creatures are most likely to follow this pattern, because temperatures in the ocean vary little from day to day.* The consequences of cold-bloodedness are often striking. During freezing weather, as much as 75% of the body water in several marine invertebrates has been found in the form of ice.943
Cold-blooded creatures are not seriously size-restricted, but tend to become lazy and sluggish in hotter or colder climes. Consequently, animals evolved certain techniques to maintain greater control over internal temperatures. It was quickly discovered that it was of tremendous advantage to be able to run the biological machinery at top speed in virtually all circumstances.
* Warm-bloodedness probably requires nonaquatic evolution. Air is less conductive than water, holds less heat, and is subject to much wider thermal variations. Air just can’t do the job water does, so a new approach is required. Warm blooded ETs will probably have evolved that ability on land.
The fact that most organisms on Earth are mostly water is itself a tremendous thermoregulatory advantage. A 75 kg adult human, for instance, may generate ten million joules of energy in a single day. As pure heat energy, this would suffice to raise the body temperature only about 30 °C. But if almost any substance other than water were used, the same quantity of heat would cause a temperature rise of from 100-150 °C — which is excessive.
| Dilating or constricting blood vessels |
Aliens, like terran lifeforms, may also control body heat by dilating or constricting blood vessels in the skin. An extraterrestrial with green blood whose body is overheating may appear flush-skinned, a sickly-looking pale patina.
| Thermal radiators and cooling vanes |
There are variations on this theme. Elephants are thought to use their giant ears as great thermal radiators. There is much speculation that the tough armored dorsal fins of the extinct dinosaur Stegosaurus might have acted as cooling vanes. ETs may come up with similar adaptations, especially among the larger creatures and in hotter planetary environments.
| Evaporative cooling |
Evaporative cooling is another simple technique. Humans can sweat out as much as 10-12 liters of water each day from some six million evaporative sites in the skin. However, it is believed that only large warm-blooded organisms can afford to throw away the copious amounts of water needed for strictly evaporative cooling. We might also expect that aliens on very humid worlds would be far less likely to evolve evaporative thermoregulation, because high humidity means slow vaporization.
| Panting, saliva spreading and wallowing |
Again, there are variations. Some animals, such as the rabbit, the ostrich and barn owl, and the dog, are cooled almost entirely by panting.944 Saliva spreading is the only known technique among rodents, several marsupial species, bats (Megachiroptera), and has been observed in cats, elephants, and opposums.942 Wallowing is most effective in bare-skinned or sparsely-coated species. The domesticated pig cannot sweat at all, but by wallowing in mud or its own urine it can increase evaporative heat loss by as much as two orders of magnitude.941 (Intelligent wallowing ETs, the utods, appeared in Brian Aldiss’ science fiction novel The Dark Light Years.226)
| Biological electric blanket |
The Canadian harp seal is a warm-blooded animal living in extreme cold, and has evolved a unique internal heating system. It has the equivalent of a biological "electric blanket" under the skin, which can be turned up or down as required to maintain stable body temperatures. The seal can also regulate its own heartbeat, ranging from 120 down to 20 beats per minute, to change the distribution of body heat.2408
|
Other control methods:
|
Many other methods of temperature control exist among Earthly fauna which could serve as models for ETs.
- Heat can be generated by shivering.
- Many believe that feathers may have been a thermoregulatory adaptation.1653
- There is "behavioral thermoregulation" (sun-basking, shade-cooling).
- "Social thermoregulation" (school swimming in fish, hive fanning in social insects).
- "Seasonal thermoregulation" (woodchucks and marmots are warm-blooded during one season, cold-blooded in another).
- And even variable metabolic heating demonstrated in some species of plants.
| Biological refrigerator |
To the best of our knowledge there exist no organisms on Earth that directly utilize the principle of the heat pump — which Carl Sagan has aptly described as "biological refrigerators."630 The cyclical expansion and contraction of a volatile coolant fluid might be possible. Or, cycles of complementary biochemical reactions could be designed to cool an extraterrestrial lifeform at one end while radiating off heat at the other.
For instance, consider a 100 kg alien with a normal body temperature of 50 °C. If it is sitting in an environment held at a uniform 75 °C, the desired body temperature can be maintained if the creature holds one fifth of its total body mass (about 20 kg) at 175 °C. Such a heat pump mechanism would be greatly simplified if a simple cooling fluid with high heat capacity could be found, especially on a planet with an atmosphere rich in heat-conductive gases such as hydrogen or helium.
|
The Square-Cube Law:
|
The overall problem of heat management has some interesting consequences as regards the limiting sizes of organisms elsewhere in the Galaxy. As dictated by the Square-Cube Law, an animal which is twice as tall has eight times more mass — and thus eight times more waste heat is generated — but only four times as much surface area across which to radiate it off.
The problem of very large animals, then, is to find ways to get rid of lots of excess heat. Since the quickest and simplest way to do this is by conduction in water, this may explain why the largest warm-blooded animals on Earth — the whales — are found in the sea.
The largest terrestrial land lifeforms in hot, tropical climates (rhinos, hippos and elephants) are designed without body hair to facilitate heat loss. Dinosaurs are believed by many to have been hairless, and this fact may have contributed to their extinction during the periods of extensive glaciation and global cooling which followed the balmy Jurassic a hundred million years ago)142,2412
|
Whereas a human consumes only 1-2%
of his body weight in food each day, a mouse must eat 50% of its weight daily to survive. A warm-blooded animal much smaller than a mouse seems virtually impossible. |
The Square-Cube Law predicts exactly the opposite problem at the low end of the size scale. An animal which is only half as tall generates only 12½% as much waste heat but has 25% as much skin surface to radiate it away. It must eat twice as fast just to stay as warm as before. The problem of small animals (say, 1-1000 grams) is to conserve body heat and find enough to eat to stay alive. As Isaac Asimov once remarked, "no one has ever seen a fat shrew or ever will."2409
For example, whereas a human consumes only 1-2% of his body weight in food each day, a mouse must eat 50% of its weight daily to survive. A warm-blooded animal much smaller than a mouse seems virtually impossible.
|
The size and shape of extraterrestrial organisms will be
closely determined by their need for energy, the efficiency of their metabolisms and ecology, and the nature of their internal temperature control mechanisms. |
The disadvantages of small size with warm-bloodedness further aggravated in cold Arctic climes or in large heat-sink media such as the ocean. In both these niches on Earth larger animals tend to predominate — polar bears and seals in the cold North, and dolphins and whales in the seas. There are no reptiles or amphibians, and few small mammals. It is also known that body limbs and tails tend to be shorter in colder climates, a phenomenon known as Allen’s Rule.
The size and shape of extraterrestrial organisms will be closely determined by their need for energy, the efficiency of their metabolisms and ecology, and by the nature of their internal temperature control mechanisms.